When I first started researching midlife health, I never imagined how many holes I’d find in the research.
There were stacks of papers on hormone therapy, but very little on the topics that I felt could affect women’s health at this stage.
To say I’d like to see more and targeted research in women’s midlife health is an understatement. My research Wish List has grown so long I believe it’s time to share it. Of course, I won’t share everything but thought it was time to discuss what I believe is missing.
This also helps me articulate my thoughts as I contact researchers.
Menopause focus: Hormone therapy
Since the 1960s, research for midlife women has focused on menopause, hormone therapy, and related disease risk. Due to observational findings, HRT was thought to reduce the risk of heart disease, so doctors gave it to women as prevention.
Intervention studies questioned this premise, including the infamous women’s health initiative in 2002. This could be due to the use of synthetic estrogen and progestins, but a 2022 review also shows conflicting evidence for body-identical hormone therapy.
I'm following the Kronos Early Estrogen Prevention Study (Keeps) cohort closely. It includes healthy women within three years of menopause and it has three arms: Estradiol patch, oral estrogen (conjugated equine estrogen), and a control. All the women who need it are on bio identical progesterone.
After 4 years, both estrogen formulations helped reduce symptoms but did not show a significant impact on cognitive, heart health, and skin outcomes.
The data did show that those with the APOE-4 gene known to increase the risk of dementia had lower amyloid build up. Another 2023 study found that estrogen users who were APOE-4 carriers did better on memory tests and had larger brain volume.
I don’t think we need another study to see if hormone therapy is safe and effective. But I do think we need to figure out if there are some groups – like those with APOE-4- who benefit.
Could some women have trouble making estrogen?
After menopause, women primarily make estrogen from help from the adrenals. This is called intracrinology, which does not show up on blood tests because it’s made on the spot. It’s kind of like how a snap-chat message disappears. With help from the enzyme aromatase, women convert androgens, like DHEA, to estrogen the body needs, as explained in this book.
Contrary to classical endocrinology where specialized glands distribute active hormones to all tissues of the body, intracrinology has equipped each cell within each tissue with a cell-specific set of enzymes able to make intracellularly the locally required small amounts of estrogens and androgens.
A 2013 study by Lasley and colleagues, discusses the wide-women difference in the production of not only DHEA but adiol, both of which are converted to estrogen. Among women, levels of adiol can vary 10-100 fold!!
individual women have different trajectories in the rise of adrenal steroids…Because the maximal rise in DHEA and Adiol varies broadly among women, it seems possible that the effect of the very high levels may have a different effect than those of the lower levels
Some women can simply make estrogen more efficiently than others due to their DHEA and adiol levels. Could this be why some women who start taking estrogen feel worse? I would love to see more research on this because it could lead to blood tests to help clinicians determine who will do well on hormone therapy.
Breast cancer, alcohol and hormone therapy
In my post on alcohol and midlife women, I ran across surprising research about alcohol and hormone therapy. Apparently, studies since 1990 haven shown the link between breast cancer and alcohol intake is much higher in women taking hormone therapy.
A 2015 study pooled two cohorts of 30,789 women aged 50 and older. Weekly alcohol consumption of >7 drinks per week resulted in 72 additional breast cancer cases per 100,000 compared to abstainers. But for those on MHT, this number more than doubled to 180 additional cases.
Then there’s the Copenhagen City Heart Study revealing MHT users who reported more than two drinks a day had five times the risk of breast cancer compared to those who did not drink or use hormones. This isn’t new either as studies as early as the 90s showed this.
For instance, MHT users drinking alcohol increased their estrogen three-fold for about five hours with no increases seen in women not taking hormones. And a review back in 1999 concluded: “Alcohol and hormone exposure together may act synergistically to create increased breast cancer risk.”
Shouldn’t every study on breast cancer and alcohol, ask women if they are hormone therapy? And should every study on hormone therapy and breast cancer ask women about their level of alcohol consumption?
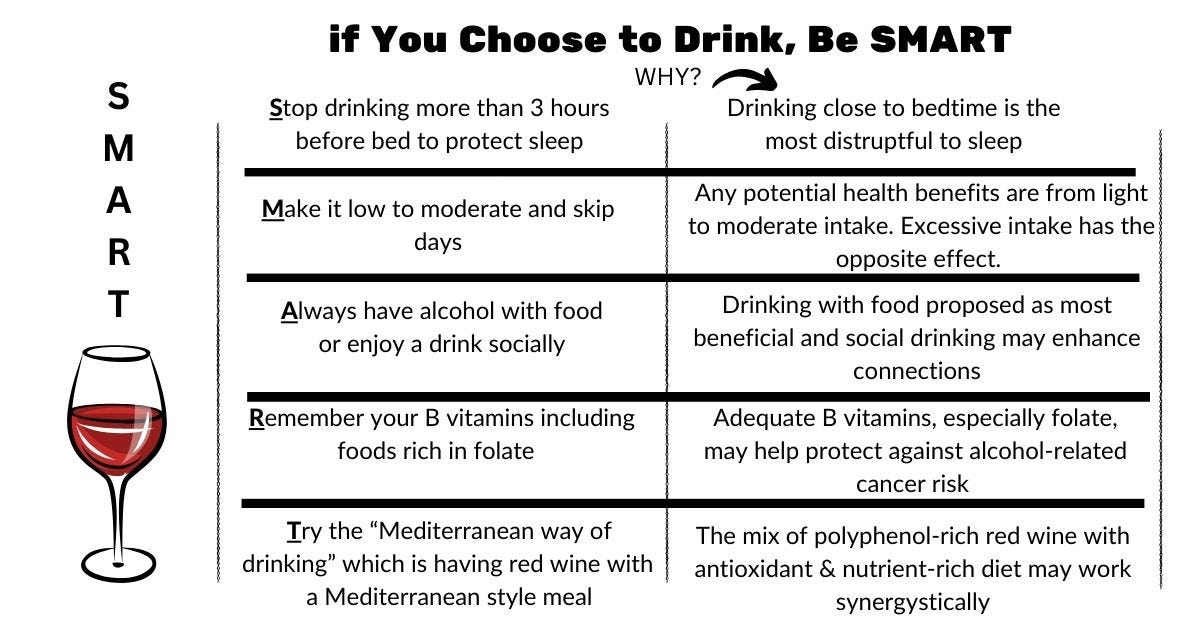
We certainly need more data to understand this combined risk so women on hormone therapy can be informed!! For now, don’t miss my SMART guidelines and the post full of the research I found.
The new needs of midlife women
Research is all about asking the right questions. Until now, questions have focused on hormonal decline during menopause, and what happens when we do or don’t add them back in.
But a developmental focus would flip this. In case you’ve missed it, I’ve explained before how midlife is a developmental stage that is missing here and here.
So what if researchers started asking: Does an aging woman moving from being fertile to infertile have different needs?
I’ve already reviewed the key micronutrients that change when estrogen declines. Isn’t it time we dedicate more research to how these nutrients impact women’s health? Both pre and post-menopausal women are missing key nutrients in their diet, but the impact is greater during and after menopause.
Choline is the most intriguing of these nutrients. Arizona State University researchers have been studying choline’s effect on Alzheimer’s in mice, finding choline supplementation greatly reducing risk throughout life.
“The research highlights a constellation of physical and neurological changes linked to choline deficiency,” reported in ASU News. “Sufficient choline in the diet reduces levels of the amino acid homocysteine, which has been recognized as a neurotoxin contributing to neurodegeneration and is important for mediating functions such as learning and memory, through the production of acetylcholine.”
There’s homocysteine again. Through its conversion to betaine, choline is involved in homocysteine metabolism. Homocysteine increases over the menopause transition. And when estrogen declines, so does a woman’s ability to make choline through the PEMT enzyme.
Not only that, but some people have genetic mutations that decrease their PEMT activity. That means they may have choline needs much higher than the recommended amount.
So where is the research on choline in midlife women? I found one study showing that supplemental choline reduced homocysteine in midlife women, but it did not evaluate cognitive status or anything else.
A 2015 study found high homocysteine resulted in lower levels of executive functioning, complex attention, cognitive flexibility, and memory in women. Not only that, but women with APOE-4 gene were most likely to have high homocysteine.
Could this be why these women do better on estrogen as mentioned earlier?
Adding to the story, choline deficiency increases the risk of non-alcoholic fatty liver disease (NAFLD). In this 2012 study, only in postmenopausal women was low choline linked with increased fibrosis in NAFLD. The researchers summarize it this way:
Our study also found that PEMT expression decreased with increasing stage of fibrosis, indicating that postmenopausal women with low estrogen levels may be at a higher risk of disease progression due to reduced PEMT expression.
Where are the choline studies on midlife women examining cognitive health, liver health, and genetic variations?
We are the population researchers need to study!
Vasomotor symptoms as a risk factor
I think it’s safe to say that vasomotor symptoms such as hot flashes, mood disorders and poor sleep are risk factors for diminishing vascular health. Yes, low estrogen triggers it. But not all women experience symptoms–or the same severity of symptoms — and they are not related to blood levels of estrogen.
Something else is going on that predisposes women.
This is where aging comes in. Nitric oxide, the magic midlife molecule, is roughly half of what it was in our 40s compared to our early 20s. Because both estrogen and progesterone stimulate nitric oxide synthesis, menopausal women are hit hard.
We still do not know exactly why hot flashes occur. The primary theory is declining estrogen disrupts the body’s thermostat (hypothalamus). But this is too vague for me.
Since learning about nitric oxide, I’m very interested in it as a possible link to hot flashes, sleep and many of the health issues midlife women face. One theory is that because nitric oxide is released when a hot flash occurs, blocking it might help.
Two studies used nitroglycerin patches and the first (shorter one) showed a reduction in hot flashes but the second (longer one) did not. The body builds up a tolerance to nitroglycerin and eventually bocks nitric oxide, which is why nitroglycerin is not a long-term therapy.
But if estrogen and progesterone help stimulate nitric oxide, why would blocking it help with hot flashes? I mean, it could help stop a hot flash, but this would not be good for our health. The same way wearing nitroglycerin is not a long-term solution.
A 2017 study offers yet another explanation for hot flashes, and this has to do with nitric oxide-induced heat shock proteins. Nitric oxide increases heat shock proteins, which have many health benefits. And it seems heat shock proteins also increase nitric oxide. The theory is that when nitric oxide plummets with hormonal declines, the body’s heat shock response (HSR) gets thrown off and the body gets hot to compensate.
It is intriguing that all menopausal women have low circulating oestrogen levels, yet not all of them exhibit hot flushes. a member of the 70 kDa family of heat shock proteins (HSP70), which are anti-inflammatory and cytoprotective (i.e. cardioprotective, vasculoprotective, neuroprotective, antiatherosclerotic and antidiabetic) protein chaperones whose expression is modulated by different types of physiologically stressful situations, including exercise and heat stress Hence, it is not surprising that, during perimenopause, several homoeostatic functions based on oestrogen-dependent HSP70 expression start to collapse.
I found a follow up to this in a study done with mice. Estrogen-deficient mice that were heat treated reestablished their HSR pathway and recovered metabolic function. “Reinforcing the proposal of thermal therapy as a strong alternative to hormone replacement therapy whose long-term safety is questionable.”
Raising core body temperature is one key way to induce heat shock proteins. In this review she published last year, Rhonda Patrick reviews the research on sauna use and its benefits. Sauna use improves sleep, decreases the risk of heart disease, reduces the risk of depression, helps maintain muscle mass, and reduces the risk of neurodegenerative diseases like Alzheimer’s.
A Finnish pilot study revealed that women who had a series of sauna sessions had significantly less symptoms including night sweats, palpitations, sleep problems, and irritability. And a 2011 intervention study showed post-menopausal women who had local thermal therapy (two-20-minute sessions per week) with far-infrared had significant reductions of menopause-related symptoms.
What’s interesting is that immersing yourself in hot water, like with baths and hot tubs, may have similar benefits. According to a 2019 review on the subject:
the physiological changes induced by warm water immersion, such as vasodilation, increased blood flow, reduction of arterial stiffness, vascular endothelial function, oxygenation, and decreased sleep-related stress, may result in improvements in the cardiovascular function. These physiological changes due to water immersion are similar to the cardiovascular effects of physical activity
It’s time to change directions
Why aren’t we doing more studies on sauna or thermal therapy on midlife women? Although raising core body temperature is one way to increase NO and HSR, there are many others.
These strategies not only may help hot flashes, they stand to improve the health risk that go with aging and menopause. Estrogen will help some women, but research is mixed on its risk reduction.
Could some women simply not make enough estrogen after menopause? Is choline the key to brain fog and women's higher risk of Alzheimers? How would meeting other at-risk micronutrients stand to help midlife women? Could boosting nitric oxide help not only with vasomotor symptoms but health and quality of life?
These are all questions I would love to see answered. We don’t need another study on hormone therapy unless it’s going to give us new information.
It’s time we take a developmental approach and find actual answers once and for all. Midlife women deserve that, don’t you think?








All great questions as usual. :) And this may be slightly off topic, but what about testosterone replacement therapy in midlife women as well? I keep hearing about how T hormone levels are also very important in women (albeit in much less amounts than men) for energy, libido, etc... but there is virtually no reliable research on how much, best absorption delivery, how often for midlife women, breast cancer/clotting risk factors and if T should given alone or with estrogen + progesterone is appropriate. And unless you live in a big city, hardly any HRT specialists are available to meet/discuss solutions and safety of HRT or just TRT for menopausal women, and even when you find one-- it's big $$$! It's all just so frustrating for us women who are in midlife looking for relief!