
“Maryann is a delightful girl, but she asks too many questions.”
When I look back on my grade school report cards, that comment from one of my teachers has stuck with me.
Granted, asking questions at a young age was partly because of my anxious nature. But this quality remains true to this day. In midlife health for women, I ask lots of questions and honestly, I’m often dissatisfied with the answers. This explains my deep dive into research.
One question that keeps coming up is why do some studies show menopausal hormone therapy (MHT) increases cardiovascular risk while others show a decreased risk?
I know, I know. Aging and time from menopause are key factors. But why exactly?
Late last year I found a review that tackled this very question entitled: Estrogen and the Vascular Endothelium: The Unanswered Questions. I get so excited when I hear the words estrogen and endothelium in the same sentence.
So, I contacted the lead researcher, got the study, and devoured it. And it brought me to another study I’ll talk about in a minute. A gem no one seems to be talking about.
“The goal of this review is to explore our current understanding of the influence of estrogen on the human vascular endothelium and highlight novel mechanisms and hypotheses that may explain the apparent disconnect between estrogen supplementation and cardiovascular health.”
-SenthilKumar, Endocrinology, 2023 Apr 17;164(6)
Estrogen and macrovascular function
I’ve written about nitric oxide (NO) quite a bit, a molecule secreted by the endothelium (cells that line arteries) that helps it dilate and increase blood flow. But you know what’s sad? There is not a universally agreed upon way to measure it.
Researchers can use flow mediated dilation (FMD) of the brachial artery to determine if nitric oxide is causing vasodilation in large arteries. FMD is a reliable test for assessing the impact of estrogen and menopause on macrovascular health.
The Hunt3 fitness study compared FMD to women and aged matched men. FMD was higher in women–25% higher than men in the 20s, 27% higher in their 30s, 34% higher in their 40s and 23% higher in their 50s and 13.2% higher in their 60s, and the same at age 70. In females, there was an increase in the 15-age group, showing an effect of estrogen:
These studies support the notion that FMD, a predominantly endothelial- and NO-dependent phenomenon, is influenced by biological sex and estrogen. - SenthilKumar, 2023
FMD significantly decreases throughout the menopause transition. In postmenopausal women, FMD is 50% lower than premenopausal women, of which 35% is likely because of hormones. Preclinical and human studies show supplemental estrogen increases FMD, positively affecting large artery function.
For instance, trans women who take estrogen have similar FMDs to age-matched women. But research also shows trans women and older women who take estrogen are at higher risk for cardiac events. Why is that?
New research is showing estrogen may have a different effect on microvascular function, the very neglected small arteries.
Estrogen and microvascular function
FMD is not used to measure blood flow in small arteries or arterioles. In fact, unlike large arteries, arterioles can switch from nitric oxide to hydrogen peroxide (H202) for vasodilation. Prolonged H2O2 can create a proinflammatory and pro-thrombotic environment, which affects the one health principle for midlife women.
Long-term estrogen use in post-menopausal women improves arteriole blood flow, but only in those who are deemed healthy. What gives?
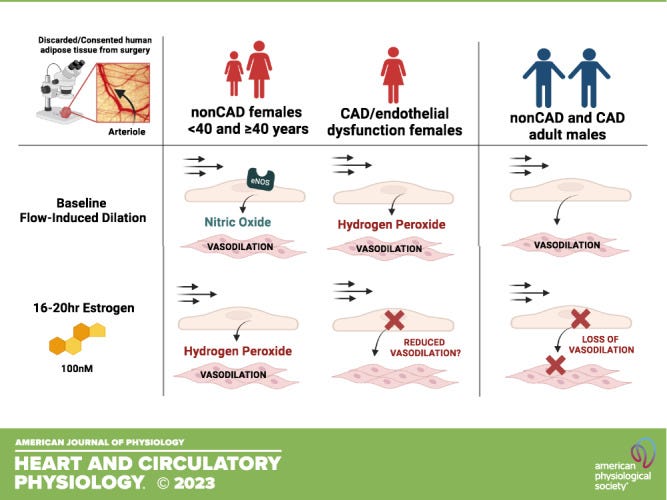
In its first-of-its kind study published in 2023, researchers used isolated human arterioles and found that females (30-78 years old) maintained NO flow induced dilation (FID). Exposure to 100nM of estrogen–considered an elevated dose–revealed a switch from NO to H202 in all the arterioles.
The women with coronary artery disease showed a trend toward reduced FID. But in cis males, there was a significant reduction in FID.
The researchers speculate that estrogen receptors may play a role, as large arteries express ER-a and ER-b, while ER-b is the only estrogen receptor expressed in the microvascular. This matters because ER-a is the receptor involved in the NO response:
“Given the predominant expression of ER-b in the human microvascular endothelium, as well as undetectable levels of ER-a, it is possible that estrogen promotes a more oxidative environment in the microvascular endothelium. Also, important to note, estrogen concentrations fluctuation in women not undergoing hormone therapy possibly allowing time for recovery.”
In an email conversation, the lead researcher, Gopika Senthilkumar, told me that this is very preliminary research, and they don’t know if this occurs in full humans. The level of estrogen, whether high or low, could be a determining factor. Same goes with continuous versus cyclical.
Why this WHY resonates
The review I mentioned explores additional reasons estrogen can cause problems, including the timing hypothesis and the effects of aging. Out of all the evidence, the impact on microcirculation is the most logical to me.
The main reason being that coronary microvascular disease is more common in women, and it’s considered the basis for other women-centric diseases like rheumatoid arthritis.
This is not just about exogenous estrogen but naturally producing levels in premenopausal women. Consider women with PCOS who have normal or high estrogen but do not always ovulate. They are also at higher risk for microvascular dysfunction. Pregnancy, and higher levels of estrogen, can also bring on complications like preeclampsia and gestational diabetes, both linked to microvascular dysfunction..
While the diagnostic criteria for preeclampsia relies on the development of de novo hypertension and accompanying clinical symptoms after 20-week gestation, it is likely that subclinical dysfunction of the maternal microvascular beds occurs in parallel and may even precede the development of overt cardiovascular symptoms in these women.
There is always this attempt to make estrogen all good or all bad. And I’m certainly not doing that. But I think it’s important to understand the nuance of why, sometimes, estrogen can be problematic in certain women, under certain circumstances. Many women don't even know that their microcirculation is compromised. We need to know who these women are and how they can avoid the risks.
One thing is clear. For hormone therapy, we need to focus more on microvascular function. Perhaps researchers can figure out the ideal type, dose, and timing that confers the most benefits and least risk. It’s hard to say because there’s so much more research that needs to be done.
“Potential mechanisms that link estrogen exposure to microvascular dysfunction represent a critical knowledge gap in our understanding of how hormone treatment may contribute to future CVD risk.”
-Am J Physiol Heart Circ Physiol. 2023 Mar 1; 324
Asking different questions
Until we get better answers, we can ask different questions.
The undeniable truth is that aging pause makes women more susceptible to endothelial dysfunction in arteries of all sizes. So I keep asking and digging to find “non-hormonal” lifestyle ways to improve this reality.
I just didn’t want to let too much time go by without mentioning this research. Nuance doesn’t make the news, and that’s unfortunate. But I find many times, some of the best studies do not get airtime at all.
Probably because it's hard to produce a soundbite, and write about.
I’ll keep being that annoying question asker, because, someone’s gotta do it.
Any thoughts on this research?




Thank you Maryann for another inciteful article. I'd be interested in links to this and Reynaud's Disease. I believe Reynaud's mainly affects women and symptoms don't normally start till the onset of puberty - so estrogen must be linked. It's also hereditary. Also intrigued by potential covid links as I know a number of people developed circulatory issues - eg chilblains (am assuming that more micro- than macro circulation at play?)
Interesting to me, after experimenting with methylene blue, which shows promise for brain cognition. The downside is, it suppresses “excess” estrogen and nitric oxide. Not a problem for a male in his twenties. But for myself at 62, it ruined my workouts and caused some issues that can only be chalked up to estrogen deficiency. Appreciate the info